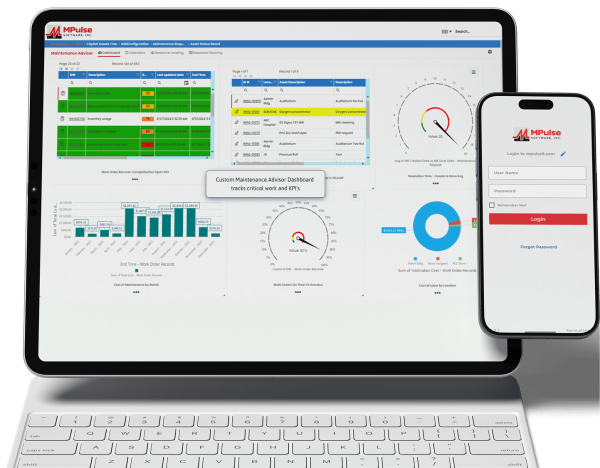
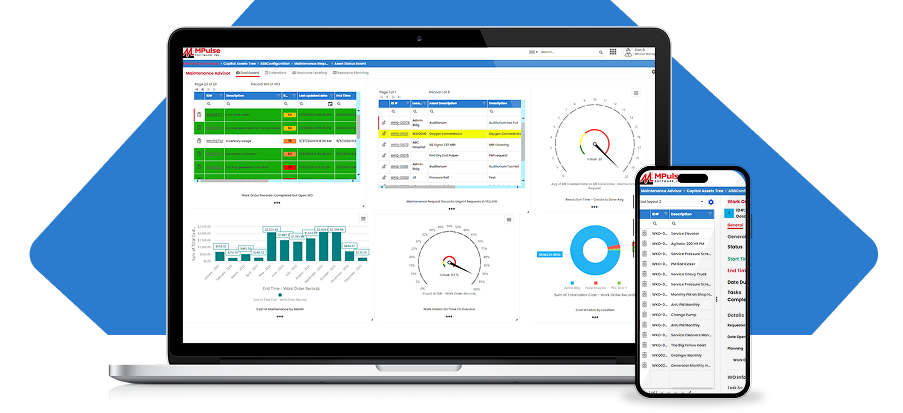
Maintenance plays a major role in keeping medical equipment running smoothly and ensuring hospitals or clinic facilities are operating efficiently. That includes continuously striving to meet the growing demands of the U.S. Department of Labor’s Occupational Safety and Health Administration (OSHA) regulations.
Luckily, they have help. CMMS software helps improve workplace safety and implement worker protections to reduce and eliminate hazards in healthcare facilities, thereby preventing future workplace injuries and illnesses.
With a properly implemented CMMS solution, your organization can pass inspections and audits, and avoid OSHA violations in healthcare facilities. And you won’t have to scramble at the last minute to set things in order.
Here’s how.
Table of Contents
OSHA Regulations for Healthcare Facilities
MPulse customers use CMMS software to comply with OSHA standards by…
- Documenting safety training
- Publishing safety data as a reminder to employees
- Standardizing checklists for scheduled inspections
- Creating a paper trail showing preventive measures
- Updating safety procedures
- Detailing emergency procedures in case of a natural disaster or other incident
Proactive action provides long-term benefits when it comes to health and safety. Here are the top OSHA violations in health facilities and how to avoid them.
COVID-19
Healthcare facilities and providers are on the front line of the COVID-19 pandemic. OSHA has issued temporary enforcement guidance related to COVID-19, which is a recordable illness if a worker is infected as a result of performing their work-related duties. Using CMMS software to log employee incidents properly and to maintain records in accordance with OSHA’s current standards allows you to present the solid documentation that OSHA demands.
Hazards
In addition to COVID-19, risks to healthcare workers include hazardous chemicals or drugs, as well as bloodborne pathogens such as HIV, Hepatitis B, and C. Employers must make information about the identities of these hazards available and understandable to workers. For example, drug manufacturers and importers are required to evaluate the hazards of the substances they produce or import, and prepare labels and safety data sheets to convey the hazard information. Additionally, employers must ensure hazardous materials are labeled, provide safety data sheets for their exposed workers, and train them to handle them appropriately.
Safety Training
OSHA requires organizations to record and track safety training for employees. CMMS software helps you track health and safety data and resolve potential risks before outside auditors do, such as who’s had what training. Additionally, automated alerts notify maintenance managers when certifications will expire.
Documentation
Inconsistent record-keeping procedures are a common cause of OSHA citations and fines. CMMS data provides both the big picture and the little details, so your maintenance tasks are both visible and verifiable. Much of that documentation is also automated. And best of all, you can access the information in minutes, instead of spending hours or even days sorting through a paper filing system.
Failure to Report
OSHA requires most employers with more than 10 employees to keep a record of serious work-related injuries and illnesses. (Certain low-risk industries are exempt, and minor injuries requiring first aid only do not need to be recorded.) Employers must report any worker fatality within 8 hours and any amputation, loss of an eye, or hospitalization of a worker within 24 hours. Failure to report serious injuries is a significant OSHA violation.
OSHA regulations for healthcare providers require documentation, particularly for creating procedures and recording maintenance activities. And there’s nothing better at documenting those tasks than CMMS software.
[related-content]
When you have solid data on your side, it makes it much easier to support your maintenance team and avoid OSHA violations. It’s in everyone’s best interest to be proactive about your employees’ health and safety.
Have questions? Contact us.